periodic table with electron configuration pdf
The periodic table organizes elements based on atomic structure‚ enabling insights into chemical properties and electron behavior. Electron configuration reveals how electrons occupy orbitals‚ shaping element characteristics and periodic trends‚ while historical contributions like Mendeleev’s and modern interpretations enhance its utility. A PDF version simplifies access to this foundational tool for education and research.
1.1 Overview of the Periodic Table
The periodic table is a comprehensive guide organizing elements by atomic number‚ showcasing their chemical properties and electron configurations. Elements are arranged in rows (periods) and columns (groups)‚ reflecting recurring patterns in atomic structure. This systematic layout helps predict behavior‚ such as valence electrons and reactivity. A PDF version enhances accessibility‚ offering a visual tool for understanding the periodic relationships and electron arrangements of elements.
1.2 Importance of Electron Configuration in Understanding the Periodic Table
Electron configuration is crucial for understanding the periodic table as it determines chemical properties and behavior. By mapping how electrons fill orbitals‚ configurations explain periodic trends like atomic radius and electronegativity. This knowledge aids in predicting oxidation states and bonding‚ making it essential for chemistry applications. A PDF version consolidates this information‚ providing a clear and accessible reference for studying element behavior and periodic relationships.

Quantum Numbers and Electron Configurations
Quantum numbers define electron orbitals‚ guiding their arrangement. The Aufbau principle ensures electrons fill lower energy levels first‚ shaping configurations that mirror the periodic table’s structure.
2.1 Quantum Numbers: n‚ l‚ ml‚ and s
Quantum numbers define electron orbitals: n (energy level)‚ l (subshell type)‚ ml (orbital orientation)‚ and s (spin). ml values range from -l to +l‚ determining orbital types. These numbers guide electron placement‚ adhering to the Aufbau principle‚ and are essential for writing electron configurations‚ which are visually represented in a periodic table with electron configuration PDF.
2.2 Aufbau Principle and Its Role in Electron Configuration
The Aufbau Principle dictates that electrons fill lower-energy orbitals first before occupying higher-energy ones‚ guiding the order of electron configuration. This principle‚ combined with quantum numbers‚ ensures electrons are placed in the most stable arrangement. It is visually represented in a periodic table with electron configuration PDF‚ helping to predict chemical properties and understand periodic trends in element behavior.
Historical Development of the Periodic Table
The periodic table evolved from early arrangements like Newlands’ Law of Octaves to Mendeleev’s groundbreaking version in 1869. His work laid the foundation for modern interpretations‚ integrating the periodic law and electron configurations into a comprehensive tool for understanding elements.
3.1 Mendeleev’s Contributions to the Periodic Table
Dmitri Mendeleev’s 1869 periodic table revolutionized chemistry by arranging elements based on atomic weight and recurring chemical properties. His innovative approach predicted missing elements and corrected atomic weights‚ laying the foundation for modern periodic law. Mendeleev’s work integrated the periodic table with electron configurations‚ enhancing its utility and accuracy for future scientific advancements and educational applications.
3.2 Modern Interpretations of the Periodic Law
Modern interpretations of the periodic law incorporate quantum mechanics‚ emphasizing electron configurations and orbital filling. The periodic table now reflects the quantum-based structure of atoms‚ with elements arranged by atomic number and recurring trends in chemical properties. Digital tools‚ such as PDF versions‚ enhance accessibility and visualization of electron configurations‚ aiding in teaching and research applications of the periodic law.
The Structure of the Periodic Table
The periodic table is structured by atomic number‚ with elements arranged in periods and groups. Electron configurations determine periodic trends‚ such as atomic radius and electronegativity variations across periods and down groups‚ reflecting the periodic law’s fundamental principles and aiding in understanding chemical behavior.
4;1 Periods and Groups in the Periodic Table
Periods represent horizontal rows‚ each corresponding to a principal quantum number‚ while groups are vertical columns sharing similar chemical properties due to the same electron configuration in their valence shells. Elements in the same group exhibit consistent valence electron behavior‚ allowing for predictable chemical interactions‚ reflecting the periodic law’s structure and electron configuration principles effectively in a PDF format.
4.2 Relationship Between Electron Configuration and Periodic Trends
Electron configuration directly influences periodic trends such as atomic radius and electronegativity. As electrons fill orbitals‚ periodic patterns emerge‚ with elements in the same group sharing similar valence configurations. Moving across periods‚ increasing nuclear charge affects electron behavior‚ while descending groups show added shells. These trends‚ visually represented in a PDF‚ help predict chemical properties and behaviors based on electron arrangements. This relationship is fundamental to understanding the periodic table’s structure and utility in chemistry.

Electron Configuration Notations
Electron configurations are written using quantum numbers and orbital notation. The standard method shows filled orbitals‚ while condensed forms use noble gas abbreviations for simplicity‚ aiding quick analysis of chemical properties and periodic trends.
5.1 Writing Electron Configurations for Elements
Electron configurations describe the distribution of electrons in an atom’s orbitals. To write one‚ identify the element’s atomic number‚ then fill orbitals in order of increasing energy‚ following the Aufbau principle. Use quantum numbers n (energy level)‚ l (subshell)‚ and ml (orbital). Electrons fill orbitals singly before pairing‚ adhering to the Pauli exclusion principle. Noble gas core configurations simplify writing by abbreviating filled shells‚ making it easier to identify oxidation states and chemical properties.
5.2 Condensed and Long Forms of Electron Configurations
Electron configurations can be written in long or condensed forms. The long form lists all electrons explicitly‚ while the condensed form uses noble gas abbreviations (e.g.‚ [Ne]) to represent filled shells. This simplifies writing configurations for elements with many electrons. The periodic table with electron configuration PDF often displays both forms‚ providing clarity and readability for students and researchers. This format highlights periodic trends and simplifies understanding of electron distribution.
Practical Applications of the Periodic Table with Electron Configuration
Electron configurations help determine oxidation states‚ identify valence electrons‚ and predict chemical properties‚ making the periodic table a versatile tool for chemists and educators. PDF versions enhance accessibility and understanding of these applications.
6.1 Using Electron Configurations to Determine Oxidation States
Electron configurations are essential for identifying oxidation states‚ as they reveal the valence electrons available for chemical reactions. By analyzing the outermost electrons‚ chemists can predict how elements lose or gain electrons‚ forming ions. This knowledge is crucial for understanding chemical bonding and reactions‚ making it a fundamental tool in both education and research settings. The periodic table’s structure‚ particularly in PDF formats‚ simplifies this process by providing a clear visual representation of electron distributions‚ allowing for quick identification of trends and patterns in oxidation states across different elements. This practical application underscores the importance of electron configurations in chemistry‚ enabling precise predictions and a deeper understanding of elemental behavior.
6.2 Identifying Valence Electrons and Chemical Properties
The electron configuration in a periodic table PDF highlights valence electrons‚ which determine an element’s chemical properties and reactivity. Valence electrons are those in the outermost shell‚ influencing how elements form bonds. By examining these configurations‚ chemists can predict properties like electronegativity‚ ionization energy‚ and periodic trends‚ enabling a systematic understanding of chemical behavior and interactions. This makes the PDF a valuable resource for both students and researchers.
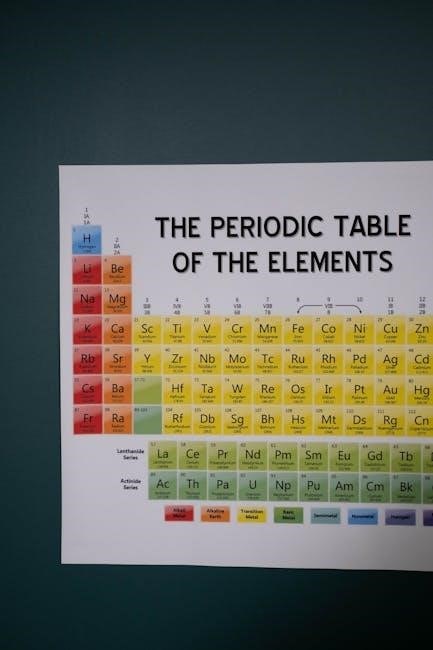
A PDF version of the periodic table with electron configurations provides a concise and portable reference. It lists elements’ atomic numbers‚ symbols‚ names‚ and detailed electron setups‚ aiding in understanding chemical properties and periodic trends. This format is ideal for easy access and printing‚ making it a valuable tool for students and researchers alike.
7.1 Features of a Comprehensive PDF Version
A comprehensive PDF version of the periodic table with electron configurations includes detailed electron setups‚ atomic numbers‚ symbols‚ and names. It highlights valence electrons‚ oxidation states‚ and periodic trends‚ providing a clear visual representation. The PDF also features color-coded elements for easy categorization into metals‚ non-metals‚ metalloids‚ halogens‚ noble gases‚ lanthanides‚ and actinides. This format enhances readability and accessibility for educational and research purposes‚ making it an essential resource for understanding chemical properties and electron behavior across the periodic table.
7.2 How to Download and Print the PDF
To download and print the periodic table with electron configurations‚ visit a reliable educational website or platform offering PDF versions. Search for “periodic table with electron configuration PDF” and select a comprehensive version. Download the file‚ ensuring it includes detailed electron setups‚ valence charges‚ and visual aids. Print the PDF on standard paper for easy reference‚ making it a handy tool for educational or research purposes.

Educational and Research Uses
The periodic table with electron configuration PDF serves as an essential teaching tool for educators and a valuable reference for researchers studying periodic trends and atomic structure.
8.1 Teaching Electron Configurations with the Periodic Table
The periodic table with electron configuration PDF is an invaluable tool for educators‚ providing a clear and structured way to teach electron configurations. It visually organizes elements‚ showing how electrons fill orbitals and relates to periodic trends. This resource simplifies complex concepts‚ making it easier for students to understand the arrangement of electrons and their implications for chemical properties and behavior.
8.2 Research Applications of Electron Configuration Data
Electron configuration data from the periodic table PDF is crucial for research in chemistry and physics‚ aiding in understanding chemical bonding‚ reactivity‚ and periodic trends. Researchers use this data to analyze quantum mechanics‚ predict element properties‚ and study electron behavior. It serves as a fundamental reference for theorists and experimentalists‚ enhancing insights into atomic structure and its role in material science and chemical reactions.
Design Considerations for the PDF
A well-designed PDF should prioritize clarity and readability‚ incorporating color coding and visual aids to enhance understanding of electron configurations and periodic trends‚ ensuring accessibility for all users.
9.1 Layout and Readability of the Periodic Table
A clear layout is essential for readability. The periodic table should be organized with proper spacing‚ legible fonts‚ and distinct sections for periods and groups. Electron configurations should be prominently displayed‚ with key details like orbital filling and valence electrons highlighted. Strategic use of white space prevents clutter‚ ensuring users can easily navigate and understand the relationships between elements and their configurations.
9.2 Color Coding and Visual Aids for Electron Configurations
Color coding enhances comprehension by differentiating element categories. Distinct hues for metals‚ non-metals‚ and noble gases provide quick visual cues. Visual aids like orbital diagrams and electron configuration charts complement the table‚ illustrating energy levels and filling order. These tools simplify complex concepts‚ making the periodic table more accessible for learning and reference purposes‚ especially in educational settings and research environments.
The periodic table with electron configurations remains a cornerstone of chemistry‚ evolving with scientific advancements. Future versions may incorporate new elements and interactive features‚ enhancing accessibility and educational value.
10.1 The Evolution of the Periodic Table with Electron Configuration
The periodic table has evolved significantly since Mendeleev’s original version‚ incorporating quantum mechanics and electron configurations. Modern versions include detailed orbital information‚ enhancing understanding of chemical properties and trends. The integration of electron configurations has provided a deeper insight into atomic structure‚ making the table a dynamic and essential tool for both education and research.
10.2 Potential Enhancements for Future Versions
Future versions of the periodic table with electron configuration PDF could include interactive features‚ real-time updates‚ and expanded data sets. Enhancements might involve visual aids like 3D orbital representations or customizable layouts. Additional information‚ such as isotopic data or historical notes‚ could also be integrated. These improvements would make the PDF more dynamic‚ educational‚ and accessible for diverse audiences and applications.
